For hemorrhoidal pathology (hemorrhoids, anal regadi, proctitis and anal itching)

The goal of treatment is to reduce symptoms
and tissue repair
-
Medical Device based on high molecular weight Hyaluronic Acid, MSM (Methyl Sulfonyl Methane) and TT OIL (essential oil of Melaleuca alternifolia).
-
Synergistic action: high molecular weight Hyaluronic Acid reduces symptomatology and intervenes in tissue repair; MSM and TT OIL block the degradation of hyaluronic acid (1, 2) and prolong its effectiveness.
-
Indicated in the anus-rectal reparative processes such as: hemorrhoids, anal regadi, proctitis, anal itching to promote and accelerate the healing of injuries or traumas resulting from surgical treatment. (2)
-
The action of QRECTAL® is scientifically documented by clinical work (Joksimovic et al.; Uptades Surg; 2012).(1)
-
PACKAGING
Rectal GEL in 30 ml tube, fitted with a multipurpose rectal applicator.
-
DOSAGE
Apply Qrectal® 2 to 3 times a day and whenever possible after each evacuation. Continue application for 1 - 2 weeks until symptomatology disappears.(2)
High molecular weight hyaluronic acid

Stabilises intracellular structures in connective tissue and interconnects collagen fibres(1)
Facilitates the development of reparative processes (2)
Performs a lubricating action by reducing: (2)
- risk of irritation
- itching
- burning
- formation of friction injuries
MSM (Methyl Sulfonyl Methane)
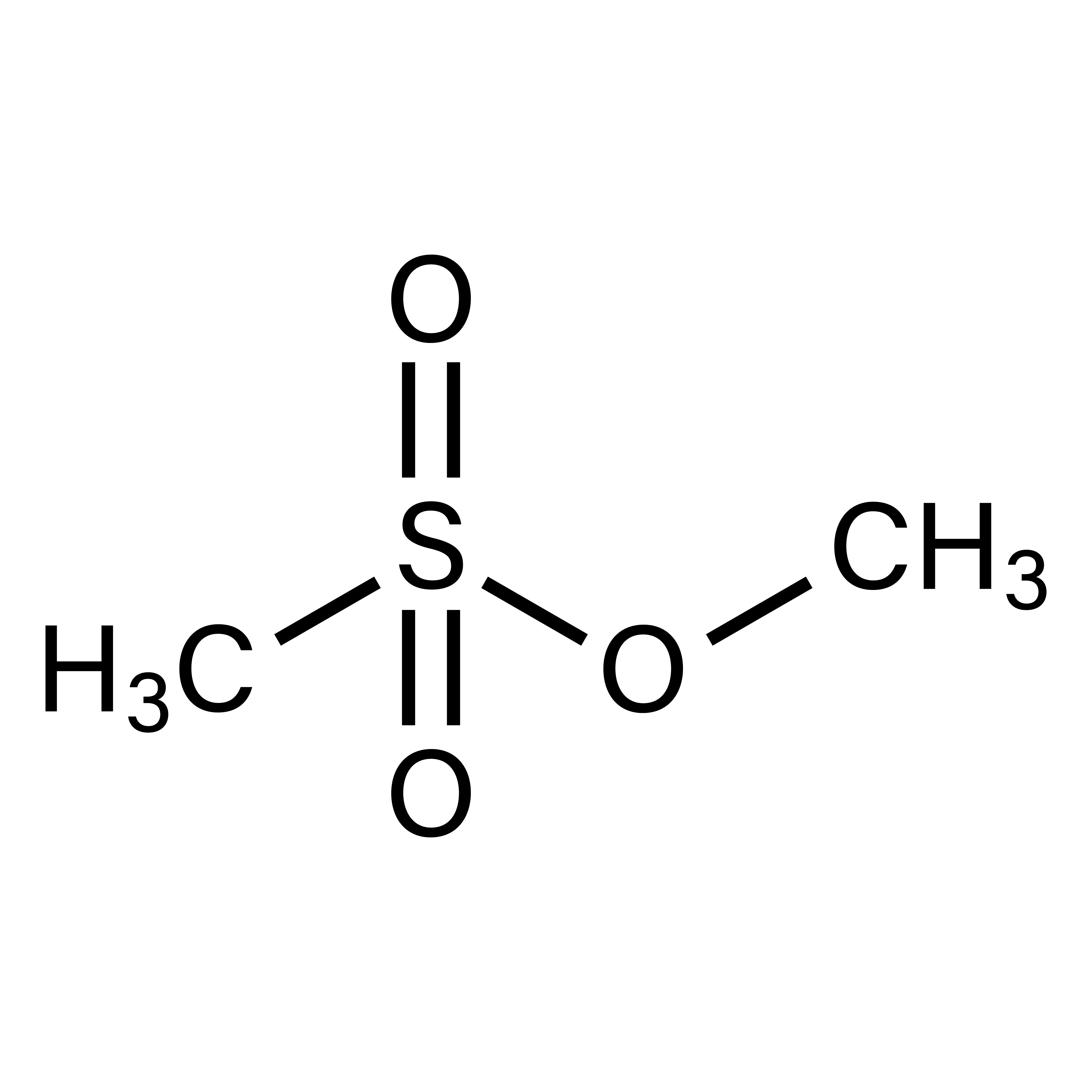
Naturally occuring in the human body (2)
Antioxidant action (1, 2)
Improved effects of hyaluronic acid (1, 2)

TT OIL (Essential Oil of Malaleuca Alternifolia)

Protective action of hyaluronic acid (1, 2)
Improved effects of hyaluronic acid (1, 2) Synergistic action between the three components that promotes the reduction of symptoms and tissue repair.

Synergistic action between the three components that promotes
the reduction of symptoms and tissue repair.
Bibliography
- Joksimovic et al. Efficacy and tolerability of hyaluronic acid, tea tree oil and methyl-sulfonyl-methane in a new gel medical device for treatment of haemorrhoids in a double-blind, placebo-controlled clinical trial. Updates Surg. 2012;64(3):195-201.
- Qrectal®. Leaflets.


